The Research Group Bioprogrammable Materials explores a young multidisciplinary field combining synthetic biology and biomaterials. It focuses on the development of materials with genetically programmed functionalities capable of biosensing, stimuli-responsive long-term drug release, and manipulation of cell behavior. Synthetic biology tools are used to program proteins and microbes to perform smart and beneficial functions. These engineered biological entities are then incorporated in appropriately developed polymeric matrices, resulting in composite materials with highly versatile functionalities, a wide range of tunability, and in situ controllability.
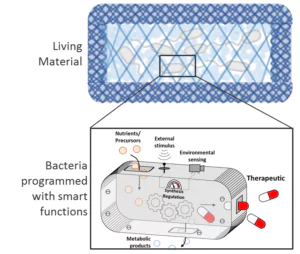
Figure: Bacterias are programmed with smart functions for applications e.g. in biosensing and drug delivery.

Staff
Research
In the Bioprogrammable Materials group, we combine genetically-programmed living organisms like bacteria with polymeric matrices like hydrogels to generate smart composite materials called engineered living materials (ELMs). Our ELMs are designed for a range of biomedical applications, such as biosensing and drug-delivery through innovations in both the living and non-living components:
Stimuli-responsive drug-secretion in bacteria
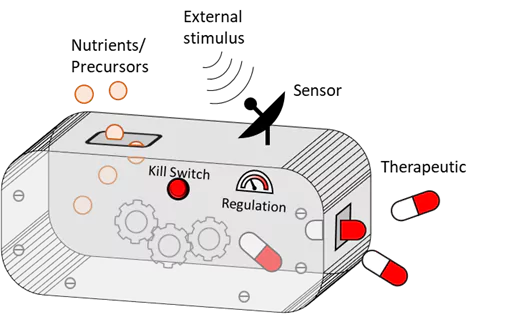
Bacteria are an integral part of the body’s microbiome, with several native and probiotic species imparting natural health benefits to humans. Bacteria are also used extensively in the pharmaceutical industry as biofactories to produce a variety of drugs. Our research merges these two features by engineering medically relevant bacteria like E. coli, Lactobacilli and Corynebacterium to produce and secrete therapeutic molecules directly in the body where they are needed. Since the bacteria naturally thrive within the body, long-term drug release can be sustained. We engineer the bacteria to produce and release anti-microbial, anti-inflammatory and regenerative drugs in the form of peptides, proteins and enzymatically synthesized biomolecules to treat chronic diseases.
Due to variabilities in patient profiles and disease progressions, it is highly desirable to personalize and customize the activity of these therapeutic bacteria in order to ensure their effectiveness. For this, we are developing genetic modules which allow external stimuli to “switch on” drug release. Stimuli like light, heat and small molecules allow remote-control over the bacteria, and stimuli like disease biomarkers will enable autoregulation of bacterial activity in response to disease progression. Stimuli-responsive genetic modules are also being developed as an additional layer of biosafety, to ensure that bacteria do not survive outside their intended implant environment. While many of the genetic modules we use were developed for E. coli, we are also endowing probiotic bacteria like lactobacilli and Corynebacterium with similar functions – a challenge which promises significant rewards by expanding the range of ELM-ready organisms and functions.
Recent publications:
- Dhakane, P.; Tadimarri, V. S.; Sankaran, S. Light-Regulated pro-Angiogenic Engineered Living Materials. bioRxiv – https://doi.org/10.1101/2022.10.28.514190.
- Dey, S.; Asensio, M. B.; Kuttae, S. B.; Sankaran, S. Novel Genetic Modules Encoding High-Level Antibiotic-Free Protein Expression in Probiotic Lactobacilli. bioRxiv – https://doi.org/10.1101/2022.08.04.502766.
Hydrogels for bacterial encapsulation and tools to understand bacterial behavior in confinement
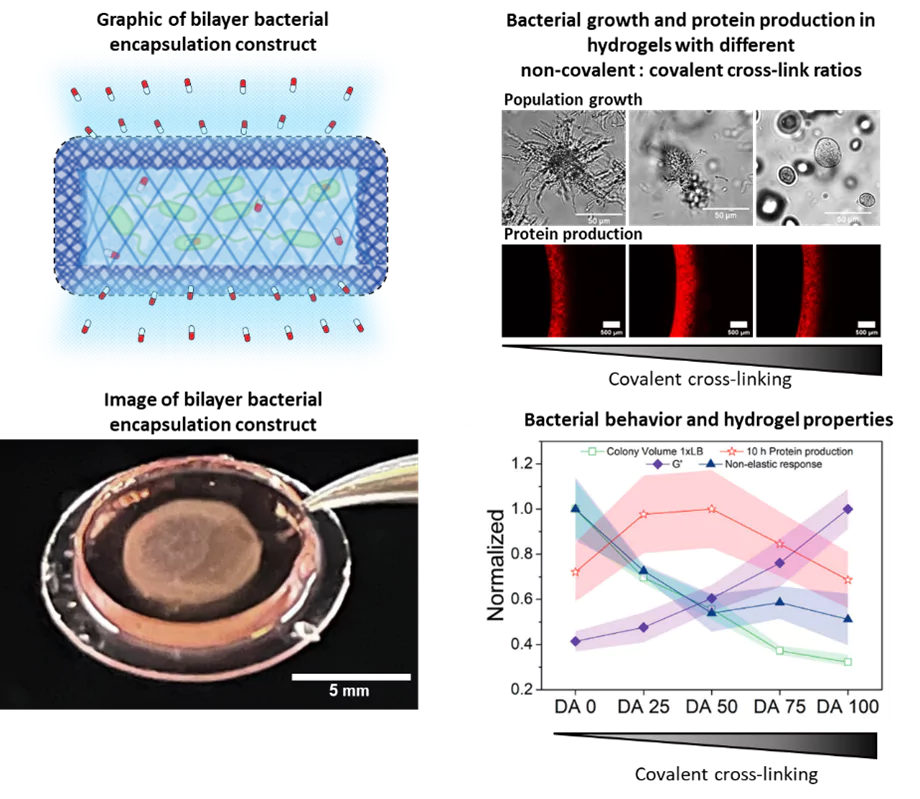
For the therapeutic bacteria to treat a disease, they need to colonize the disease site and establish a population big enough to provide effective drug doses. In collaboration with the Dynamic Biomaterials group, we are developing hydrogel matrices that provide conducive environments for the bacteria to grow and function. These hydrogels are designed to mechanically control the size of the contained bacterial population while maximizing their functionality. The hydrogel network allows diffusion of nutrients, metabolites and drugs in and out of the matrix, while preventing bacterial escape and providing protection from immune cells. The material component therefore provides an added level of biosafety for the use of genetically modified bacteria within the body.
As part of this research, we have identified that certain mechanical properties of the hydrogels influence growth and metabolism of the bacteria within, similar to how it occurs in natural biofilms. This, in turn, affects the performance of the bacteria in terms of stimuli-responsiveness and drug production. Thus, we are studying bacterial behavior confined in hydrogels, whole mechanical properties can be tuned. Thereby, we are unravelling the interplay between collective bacterial behavior and mechanical forces surrounding them. Apart from this enabling us to optimize the performance of our engineered living materials, it also serves as an artificial biofilm platform to gain fundamental insights into potential behavior of bacteria within different domains of natural biofilms. We use microscopy, biochemical and genetic assays to determine growth and metabolic behavior of the bacteria over time.
Recent publications:
Bhusari, S.; Sankaran, S.; del Campo, A. Regulating Bacterial Behavior within Hydrogels of Tunable Viscoelasticity. Advanced Science 2022, 9 (17), 2106026. https://doi.org/10.1002/advs.202106026.
Bhusari, S.; Kim, J.; Polizzi, K.; Sankaran, S.; Campo, A. del. Encapsulation of Bacteria in Bilayer Pluronic Thin Film Hydrogels: A Safe Format for Engineered Living Materials. bioRxiv – https://doi.org/10.1101/2022.09.29.510162.
Collaborations:
These research endeavors are also part of multiple collaborations within two consortia – (i) Leibniz Science Campus on Living Therapeutic Materials (LSC LifeMat) and (ii) Collective Research Center on Physical modeling of non-equilibrium processes in biological systems (CRC 1027).
Finanzierung:


Publications
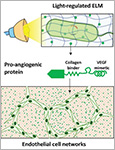
Dhakane, Priyanka | Tadimarri, Varun S. | Sankaran, Shrikrishnan
Advanced Functional Materials , 2023, 33 (31), 2212695.
https://onlinelibrary.wiley.com/doi/10.1002/adfm.202212695
Riedel, Florian | Puertas Bartolomé, María | Teruel Enrico, Lara Luana | Fink-Straube, Claudia | Nguyen Dong, Cao | Gherlone, Fabio | Huang, Ying | Valiante, Vito | del Campo, Aránzazu | Sankaran, Shrikrishnan
Frontiers in Bioengineering and Biotechnology , 2023, 11 1278062.
https://www.frontiersin.org/articles/10.3389/fbioe.2023.1278062/abstract

Bhusari, Shardul | Sankaran, Shrikrishnan | del Campo, Aránzazu
Advanced Science , 2022, 9 (17), 2106026.
https://onlinelibrary.wiley.com/doi/abs/10.1002/advs.202106026
Rodrigo-Navarro, Aleixandre | Sankaran, Shrikrishnan | Dalby, Matthew J. | del Campo, Aránzazu | Salmeron-Sanchez, Manuel
Nature Reviews Materials , 2021, 6 1175–1190.
https://doi.org/10.1038/s41578-021-00350-8

