How do cells sense mechanical forces and how do they respond to them? This question is central to understanding tissue development, homeostasis, and a wide range of diseases. Three research teams from Germany, France, and the United States have jointly developed an innovative material that allows mechanical forces to be applied to individual cell contacts in a controlled and gentle manner, while simultaneously measuring these forces with high precision. The results have been published in the renowned journal Advanced Science.
At the core of the study is a novel light-activatable hydrogel surface on which cells can be cultured. Prof. Aránzazu del Campo, Scientific Director at INM – Leibniz Institute for New Materials in Saarbrücken, explains: “Using light, we can activate tiny molecular motors within this material that exert mechanical pulling forces on the cell’s anchoring points, the so-called integrins. These play a key role in how cells perceive mechanical stimuli.”
When the system is illuminated, the molecular motors begin to rotate and transmit a defined tensile force to the cell surface via flexible polymer chains – precisely, locally, and without damaging the cells. At the same time, fluorescent particles embedded in the gel enable the quantification of forces exerted by the cells themselves on their surroundings, as their positions shift in response to cellular traction forces.
“Using this method, we were able to observe in real time how cells respond to mechanical stimuli,” says Dr. Rinku Kumar, researcher in the Dynamic Biomaterials research department at INM and first author of the study. “What is particularly remarkable is that these effects are reversible: when the light is switched off, the external pulling force disappears, allowing us to track how cells return to their original state.”
The study thus provides new insights into mechanotransduction, the process by which mechanical stimuli are converted into biological signals. The technology opens up new possibilities for systematically investigating the role of mechanical forces in diseased tissue, such as during wound healing or cancer progression, as well as for the development of new biomaterial-based therapies.
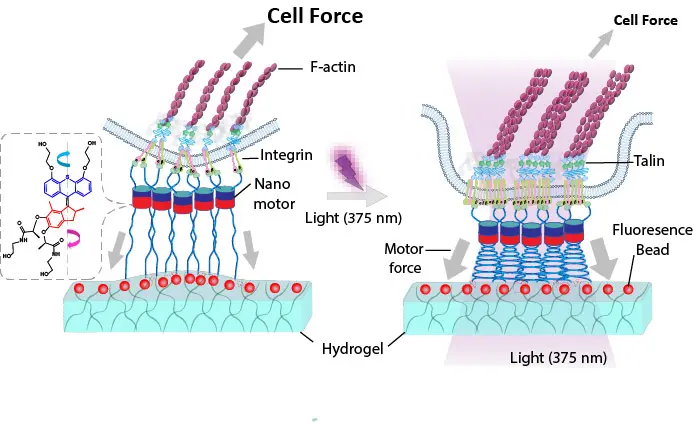
The figure shows a light-controlled hydrogel that applies targeted forces to cells. Tiny molecular motors are connected to the cell surface via flexible chains and, when activated by light, pull on cell–matrix adhesion sites. Fluorescent particles within the gel reveal the magnitude of the forces exerted by the cells, while the cells simultaneously adapt their internal actin cytoskeleton and key adhesion proteins such as talin.
Publication:
The article “An Opto-Actuated Hydrogel for Cell Mechanoactuation and Real-Time Force Monitoring” by Rinku Kumar, Marc A. Fernandez-Yague, Adrien Bessaguet, Hosoowi Lee, Nicolas Giuseppone, Andrés J. García, and Aránzazu del Campo was published on January 4, 2026, in Advanced Science

